The U.S. generic drug market is changing faster than most people realize. Between 2023 and 2025, the FDA didn’t just tweak its rules - it rewrote the playbook. If you’re a patient, a pharmacist, or someone working in pharmaceuticals, these updates directly affect what drugs are available, how quickly they reach shelves, and even how much they cost. The biggest shift? A new pilot program that rewards companies making generic drugs in the U.S.
Why the FDA Changed the Rules
It wasn’t just about efficiency. The pandemic exposed how fragile the global drug supply chain really is. Over half of all pharmaceuticals sold in the U.S. are made overseas - mostly in India and China. Active pharmaceutical ingredients (APIs), the core chemical components of drugs, are even more concentrated: only 9% come from U.S. facilities. When supply lines broke down, hospitals ran out of critical medicines like antibiotics, anesthetics, and heart drugs. The FDA had to scramble. In response, the government pushed for a strategic reset. The ANDA Prioritization Pilot Program, launched in October 2025, is the centerpiece of this reset. It’s not just another paperwork change. It’s a financial and logistical incentive: if you make your generic drug - from the API to the final tablet - in the U.S., your application gets fast-tracked.How the Priority System Works
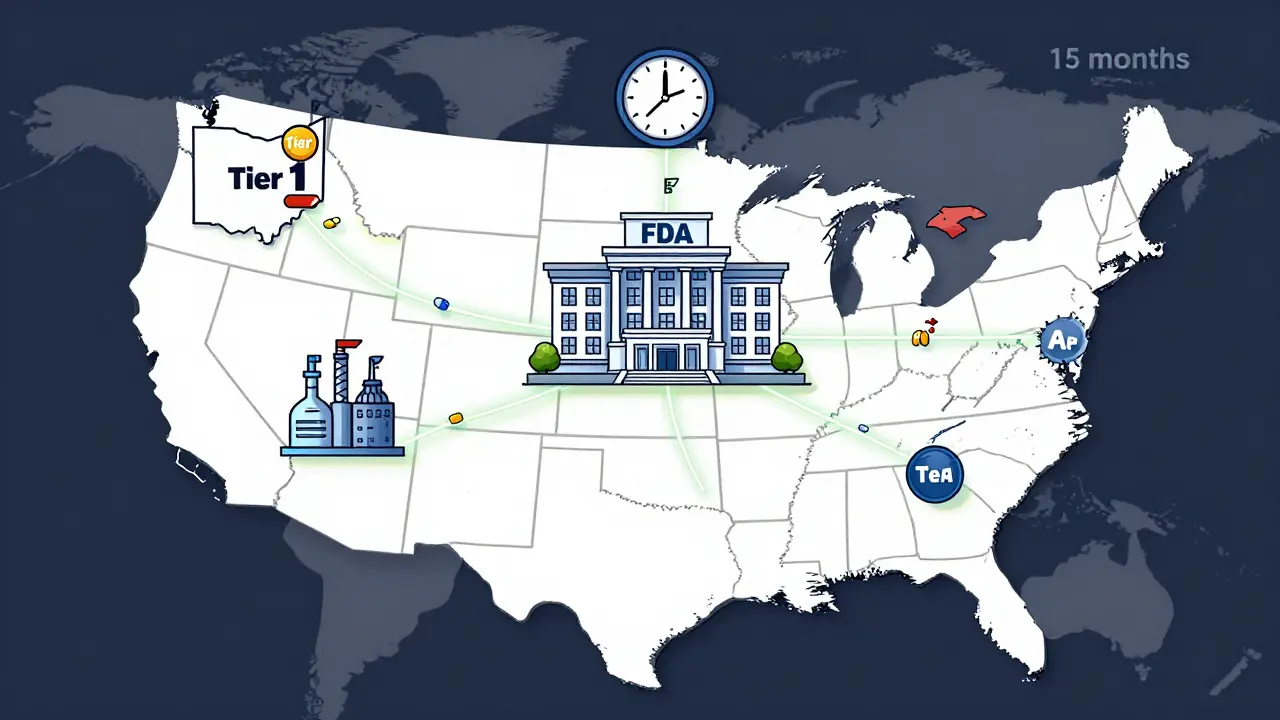
The FDA created four tiers of review speed, based on how much of the manufacturing happens domestically. The highest tier - Tier 1 - requires 100% U.S. manufacturing and testing. These applications get reviewed in just 8 months, compared to the usual 12 to 15. Even Tier 2, with 75% U.S. content, cuts review time by nearly a third. Here’s what qualifies a company for faster review:- API sourced from a U.S. facility (or one with verified equivalence)
- Bioequivalence testing done in an FDA-registered U.S. lab
- Final manufacturing and packaging completed stateside
- Proof of compliance with Current Good Manufacturing Practices (CGMP)
Who Benefits the Most?
The pilot isn’t open to every generic drug. It’s focused on medications listed on the FDA’s Drug Shortage List - currently 147 drugs - and those deemed essential by the Department of Health and Human Services. That includes drugs like nimodipine solution (used after brain aneurysms), ivermectin, and azilsartan/chlorthalidone combos for high blood pressure. Manufacturers who jumped in early saw results. Teva Pharmaceuticals brought nimodipine to market 8 months faster than expected. First generic approvals through mid-2025 were up 18.7% compared to 2024. And when a first generic hits, prices drop - on average, 78.3% within six months. But it’s not all smooth sailing. The cost to shift production to the U.S. is steep. Setting up a medium-scale manufacturing facility can cost $120 million to $180 million. Adding domestic verification to a single ANDA application adds $1.2 million to $1.8 million in upfront costs. Small companies, especially those making low-margin, high-volume generics, are struggling to keep up.
Real Numbers, Real Impact
The data tells a clear story:- Pilot applications get their first review in 30 days - half the time of standard submissions.
- Complete response letters come in 45 days, not 120.
- Approval success rates jump from 68% (foreign-made) to 92% (fully domestic).
- Major deficiency letters drop by 41%.
- Time-to-market falls from 15.6 months to 11.2 months.
What’s Still Left Out
The pilot doesn’t cover everything. Complex generics - like transdermal patches, nasal sprays, and ophthalmic suspensions - were initially excluded. These are harder to copy, require more testing, and often have fewer manufacturers. But starting January 2026, the FDA is expanding the program to include them. New guidance for these products is expected in late 2025. Another gap: API sourcing for complex molecules. Sixty-three percent of companies in the pilot say finding reliable U.S. suppliers for these ingredients is their biggest hurdle. Some have had to delay applications because no domestic source meets quality standards.Industry Reactions: Optimism With Caution
The reaction from manufacturers is mixed. On the FDA’s industry forum, many praise the faster reviews and clearer communication. Eighty-seven percent rated the FDA’s support as excellent. But on Reddit’s r/pharmaceuticals, 79% of commenters said the cost burden is crushing smaller players. A survey of 127 generic drug makers by the Association for Accessible Medicines found:- 54% have started expanding U.S. facilities
- 31% have delayed product launches
- 63% say documentation for domestic verification takes over 200 hours per application

What This Means for Patients
You might not see the paperwork, but you’ll feel the results. More first generics mean more competition. More competition means lower prices. More domestic production means fewer shortages. If you rely on a drug like metformin, levothyroxine, or amlodipine, you’re more likely to find it on the shelf. But there’s a flip side. If domestic production costs rise, insurers and Medicare may pass those costs along - at least temporarily. The Congressional Budget Office estimates the program will save $4.2 billion annually by 2030 by avoiding emergency drug purchases during shortages. But those savings won’t show up overnight.The Bigger Picture
This isn’t just about drugs. It’s about national security, economic resilience, and public health. The 2023 National Defense Authorization Act forced the government to act. Executive Order 14080 made it official: reduce dependence on foreign drug manufacturing. The FDA’s changes are bold. They’re expensive. And they’re working - slowly. The 89 applications accepted into the pilot by Q3 2025, with 27 already approved, show momentum. The FDA is also rolling out AI tools to speed up document review, promising another 25% reduction in processing time. The European Generic Medicines Association has raised concerns about trade fairness. But the U.S. government is standing firm. The Pharmaceutical Supply Chain Resilience Act of 2025, now in the Senate, would lock in these changes permanently.What Comes Next
By 2026, expect:- Expanded pilot coverage for complex generics
- More U.S. API manufacturers entering the market
- Higher prices for some generics, then stabilization
- More first generics hitting the market faster
- Reduced drug shortages for critical medications
What is the ANDA Prioritization Pilot Program?
The ANDA Prioritization Pilot Program is an FDA initiative launched in October 2025 that speeds up the approval of generic drugs if they are manufactured and tested in the United States. Companies that meet specific domestic production criteria get faster reviews - as quick as 8 months - compared to the standard 12-15 months. The program targets drugs on the FDA’s Drug Shortage List and other essential medicines.
Do these changes affect all generic drugs?
No. The pilot initially focused on simpler generics and drugs in short supply. Complex generics - like transdermal patches, nasal sprays, and certain injectables - were excluded at first. But starting in January 2026, the program expanded to include them. The FDA released new technical guidance for these products in late 2025.
Are U.S.-made generics safer than foreign-made ones?
The FDA says yes - but only because they’re more reliably produced, not because foreign-made drugs are unsafe. All generics, no matter where they’re made, must meet the same strict bioequivalence and quality standards. The pilot doesn’t lower safety requirements. It just gives priority to those who prove their entire process is U.S.-based, reducing supply chain risks.
Will generic drugs become more expensive because of this?
Initially, yes - by an estimated 12% to 18%. Building U.S. manufacturing facilities and verifying domestic sourcing adds significant costs. But experts predict prices will stabilize within 3 to 5 years as more companies invest and scale. The long-term benefit is fewer shortages, which currently cost the healthcare system billions in emergency purchases.
How can I tell if a generic drug is made in the U.S.?
Right now, you can’t easily tell from the label. The FDA doesn’t require country-of-origin labeling for generics. But if a drug was approved under the ANDA Prioritization Pilot, it’s likely made domestically. You can check the FDA’s First Generic Drug Approvals list - approved drugs from the pilot are marked with their application numbers and approval dates. Some manufacturers also advertise U.S. production on their websites.
Is this program permanent?
It’s designed to be. The FDA’s pilot is backed by the Pharmaceutical Supply Chain Resilience Act of 2025, which is currently under Senate review. If passed, it will make these prioritization rules permanent law. Even without legislation, the FDA has signaled strong support for continuing the program beyond 2026.
What should patients do if their generic drug becomes unavailable?
If your generic drug is out of stock, talk to your pharmacist or doctor. They can check the FDA’s Drug Shortage Database for alternatives. In many cases, another generic version - even if it’s not U.S.-made - may be available. Don’t stop taking your medication without medical advice. The FDA’s goal with the pilot is to prevent shortages, not create new ones during the transition.







8 Comments