When your prescription gets switched from a brand-name drug to a generic, it’s easy to feel uneasy. You’ve been stable. You know how the pill looks, how it makes you feel. Then suddenly, it’s a different color, a different shape, and the name on the bottle has changed. Is it the same? Will it still work? These aren’t just worries-they’re real concerns backed by decades of clinical research.
What Does "Bioequivalent" Actually Mean?
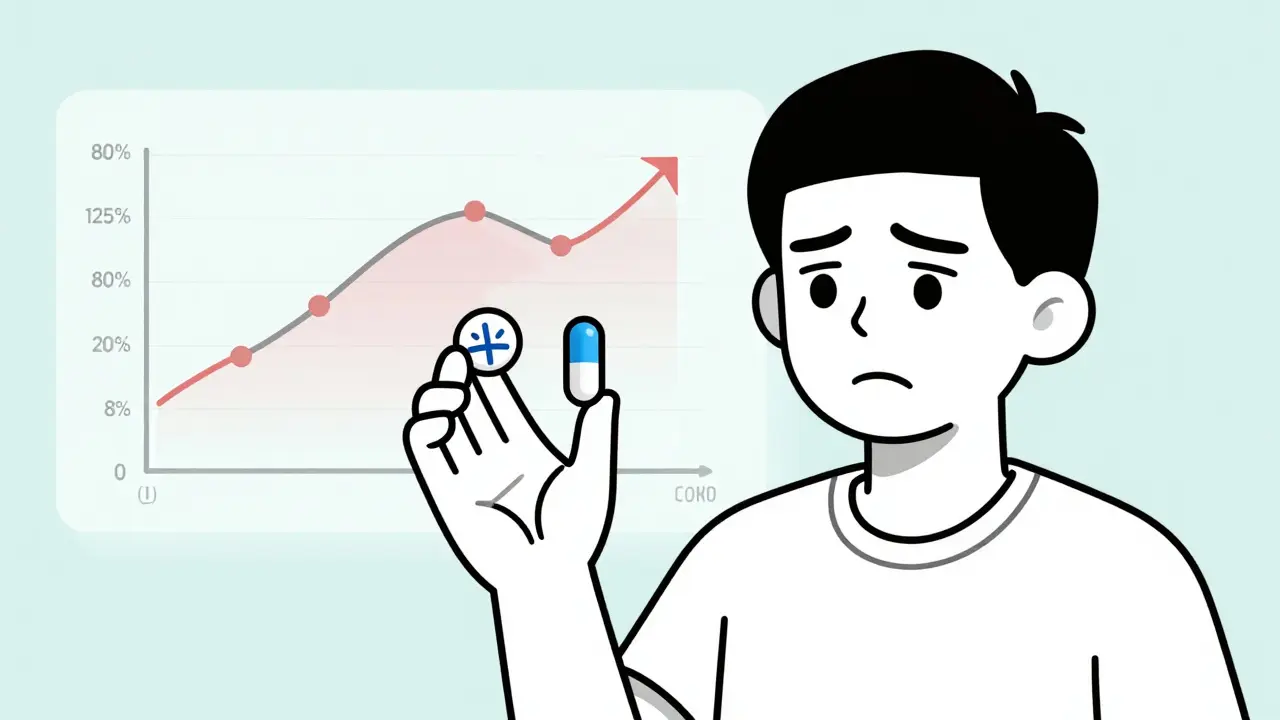
The FDA requires generic drugs to prove they’re bioequivalent to the brand-name version. That means the active ingredient must enter your bloodstream at nearly the same rate and in nearly the same amount. The acceptable range? Between 80% and 125% of the brand’s absorption levels. Sounds tight, right? But here’s the catch: that 20% variability can matter a lot if you’re taking a drug with a narrow therapeutic index. Drugs like levetiracetam (for epilepsy), phenytoin, warfarin, and some heart medications fall into this category. Even small dips in blood concentration can mean a seizure. Even small spikes can mean internal bleeding. That’s why switching isn’t just a cost-saving move-it’s a medical decision.When Generics Work Just as Well
For most people taking most medications, generics are just as safe and effective. A massive 2020 study in Nature Scientific Reports looked at 8.5 million people in Austria over five years. It analyzed 17 common drugs for heart disease, high cholesterol, and diabetes. After adjusting for everything else-age, income, other illnesses-the results were clear: generics were linked to fewer deaths and fewer major heart or brain events in 11 out of 17 drugs. For statins like atorvastatin and simvastatin, patients on generics had 15% to 22% lower risk of dying. Why? Researchers think it’s because generics are cheaper. People stick with them longer. They refill more often. Adherence matters more than the pill’s label. In hypertension, switching from brand to generic didn’t hurt outcomes-it helped. One study found patients on generic blood pressure meds were 23% less likely to stop taking their pills than those on brand names. That’s not magic. It’s money. If you’re choosing between rent and your meds, price makes the difference.The Real Trouble: Epilepsy and Antiseizure Drugs
This is where things get complicated. A 2017 review of 760 epilepsy patients showed that nearly 1 in 5 switched from brand to generic levetiracetam and had to switch back. Why? Blurry vision. Headaches. Depression. Memory loss. Mood swings. And worse-seizures. One study found that after switching to generic phenytoin, patients’ blood levels dropped by 22% to 31%. That’s not a glitch. That’s a pattern. In one group, 48.6% of patients who had breakthrough seizures had significantly lower drug levels than when they were on the brand. Their doctors didn’t change the dose. The pill just didn’t absorb the same way. The American Academy of Neurology says it plainly: most people with epilepsy can safely use generics. But some can’t. And you won’t know who until it happens. That’s why doctors who treat epilepsy often avoid automatic substitution. They want to control the switch-because the stakes are too high.What About Blood Pressure Meds? The Mixed Picture
Here’s the contradiction: one study found a 5.4% increase in emergency room visits after switching from brand to generic blood pressure meds. Another found fewer ER visits and better adherence with generics. Which is right? The difference? The first study compared two groups: people who happened to be on generics vs. people on brands. It didn’t track individuals who switched. The second study followed real people who made the switch. The latter is more reliable. But even then, not all blood pressure drugs behave the same. For bisoprolol and nebivolol, some data suggests generics might be linked to worse outcomes. No one knows why. Maybe the formulation. Maybe the inactive ingredients. Maybe the way it’s absorbed in certain people. The science isn’t settled.
Why People Switch Back-And Why It’s Alarming
A 2023 study tracked 218 patients over five years. Only 19.7% knew what their medication was for. Two-thirds identified their pills by color and shape. That’s not patient error-it’s system failure. When a pill changes appearance every time you refill, confusion sets in. One patient might get a white oval from Manufacturer A. Next refill? A blue round one from Manufacturer B. Then a yellow oval from Manufacturer C. All the same generic. All legally approved. But to the patient? Three different drugs. That’s why 12.8% of patients in the study switched back from generic to brand. Not because they felt worse. But because they didn’t trust the change. And 1.1% of patients switched between five different generic manufacturers over five years. That’s not a prescription. That’s a lottery.Who Decides the Switch? And How?
In the U.S., pharmacists can swap brand for generic without asking your doctor-unless you opt out. That’s automatic substitution. In the EU, the doctor has to write "do not substitute" if they want to keep you on brand. That’s therapeutic switching. The difference matters. When a pharmacist switches your pill, you might not even know. When your doctor changes your prescription, you talk about it. You ask questions. You prepare. Studies show that when doctors lead the switch-explain why, check in after, monitor levels-patients do better. When it’s silent, automatic, and hidden in a pharmacy receipt? Problems rise.What Should You Do?
If you’re on a medication with a narrow therapeutic index-epilepsy, heart rhythm, blood thinners, thyroid-don’t assume the switch is safe. Ask your doctor:- Is this drug known to have issues with generic substitution?
- Can we check my blood levels before and after the switch?
- Can you write "dispense as written" or "do not substitute" on the prescription?

Cost vs. Risk: The Hidden Price of Saving
Generics saved the U.S. healthcare system $370 billion in 2023. That’s real. But for epilepsy patients, the cost of a single breakthrough seizure-ER visit, ambulance, possible hospitalization-was $1,850 in 2013 dollars. Multiply that by thousands of patients. Suddenly, the savings aren’t so clear. The goal isn’t to stop generics. It’s to use them wisely. For some drugs, the system works. For others, it’s a gamble. The research doesn’t say generics are bad. It says: know your drug. Know your risk. Don’t let a pharmacy decision replace a medical one.What’s Changing Now?
The FDA is updating its rules. In 2023, it released draft guidance asking for stricter testing for certain antiepileptic generics. The European Medicines Agency now warns doctors to watch patients with unstable epilepsy, multiple meds, or liver problems. Researchers are finding genetic differences-some people’s bodies break down generics differently based on their DNA. This isn’t about fear. It’s about precision. Medicine is moving away from "one-size-fits-all" and toward personalized care. That includes how we handle generics.Final Takeaway
Most people can switch to generics without a problem. For many, it’s the only way they can afford to stay healthy. But for a subset-those on high-risk medications-switching without oversight can be dangerous. The data doesn’t give a simple yes or no. It gives a map: know your drug. Know your body. Talk to your doctor. Don’t let cost savings override safety. Because sometimes, the cheapest pill isn’t the safest one.Are generic medications always as effective as brand-name drugs?
For most medications-like statins, blood pressure pills, and diabetes drugs-yes. Clinical studies show generics perform just as well. But for drugs with narrow therapeutic indices-such as antiepileptics, warfarin, or thyroid meds-small differences in absorption can lead to real clinical problems. Not all generics are equal across all drug classes.
Can I ask my doctor to keep me on the brand-name drug?
Yes. You can ask your doctor to write "dispense as written" or "do not substitute" on your prescription. This legally prevents the pharmacist from switching you to a generic without your doctor’s approval. This is especially important if you’re on a medication with a narrow therapeutic index or have had problems with generics in the past.
Why do generic pills look different each time I refill?
Different manufacturers make generics, and each uses different inactive ingredients, colors, and shapes to distinguish their product. While the active ingredient is the same, the physical appearance changes. This can confuse patients and lead to mistakes-like thinking they’re taking a new drug or doubling up. Always check the label and ask your pharmacist if you’re unsure.
Should I get my blood levels checked after switching to a generic?
If you’re taking a drug with a narrow therapeutic index-like phenytoin, levetiracetam, warfarin, or lithium-yes. Blood level monitoring before and after switching can catch absorption problems early. Many doctors skip this, but it’s one of the best ways to ensure the switch didn’t affect your treatment.
Why do some studies say generics cause more side effects, while others say they’re better?
It depends on the study design. Studies that compare people who happen to be on generics vs. brand names can be misleading. Studies that track the same patients before and after switching show more accurate results. Also, generics often improve adherence because they’re cheaper, which leads to better outcomes overall. But for high-risk drugs, the risk of absorption variability can outweigh the benefit.





15 Comments