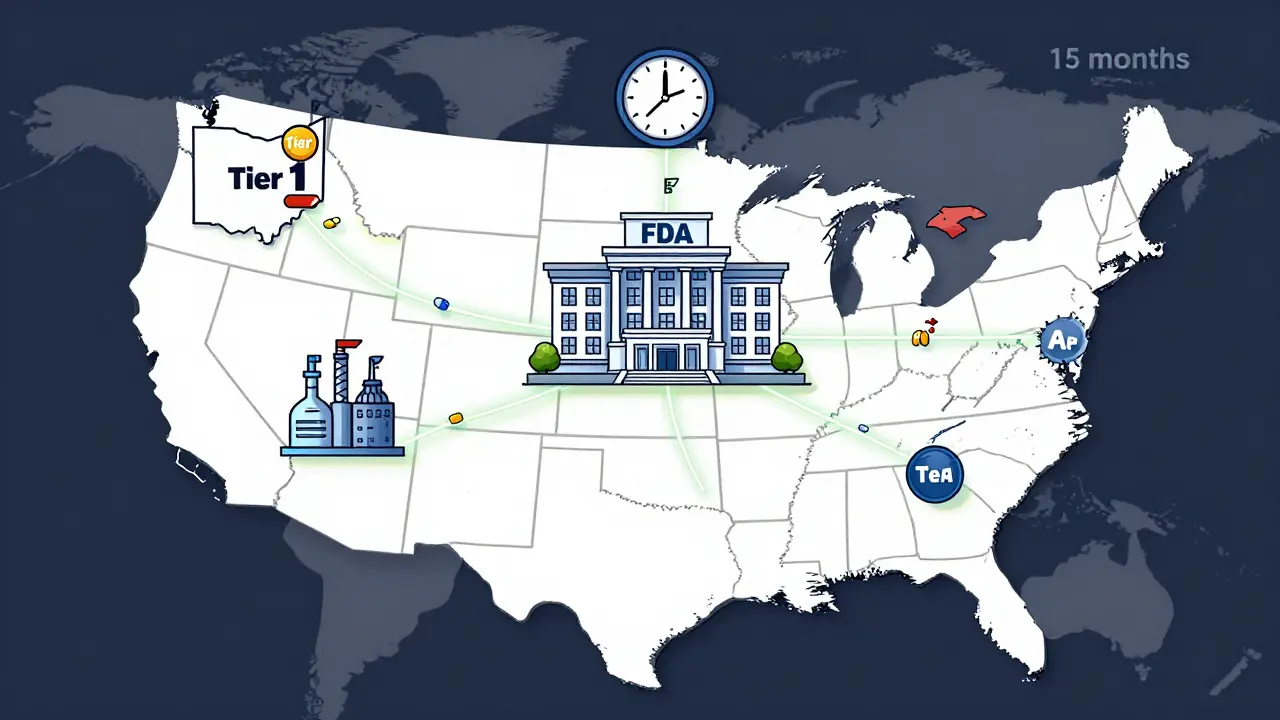
The FDA's 2023-2025 updates to generic drug approvals prioritize U.S.-made medications to fix supply chain gaps. Learn how the new pilot program affects drug availability, pricing, and manufacturing.
Tag: FDA drug shortages
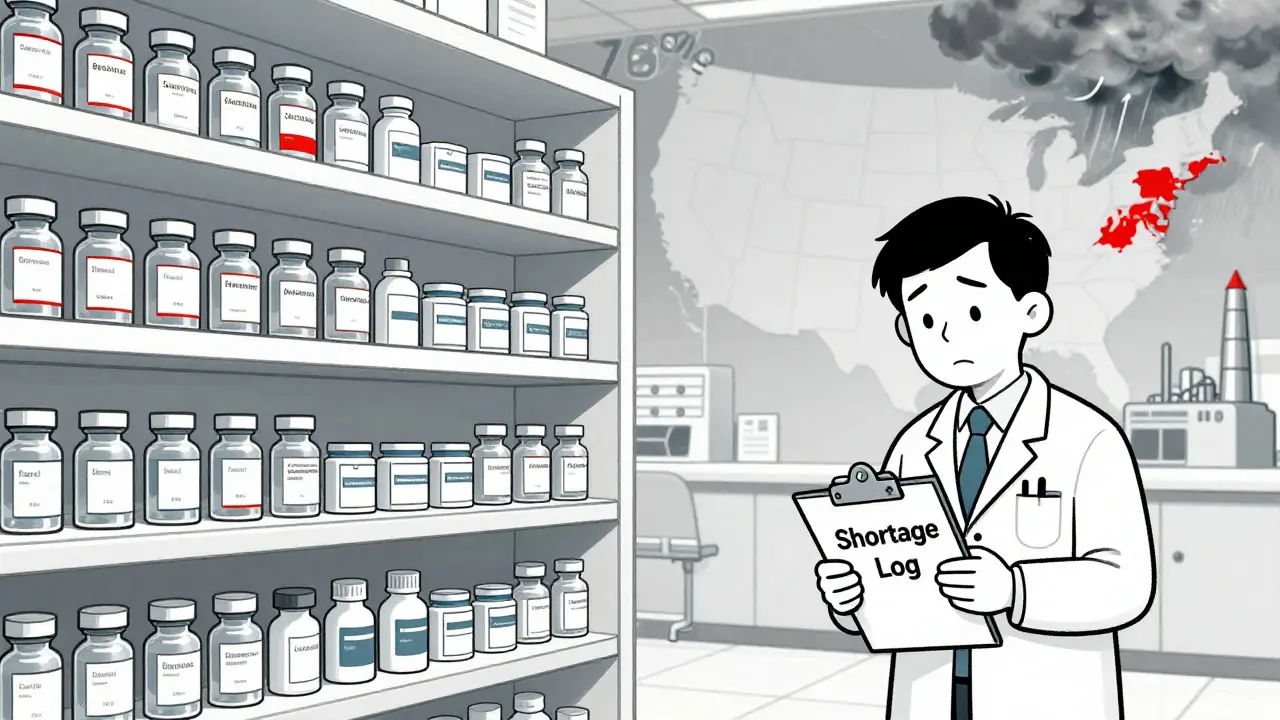
Federal actions in 2025-2026 aim to combat record drug shortages through API stockpiling, AI forecasting, and second-source manufacturing-but gaps in funding, reporting, and economic incentives still leave patients at risk.
The FDA extends expiration dates for critical drugs during shortages to keep life-saving medications available. Learn how it works, which drugs qualify, and how hospitals use this tool safely.