Every time you pick up a prescription, there’s a good chance the pill in your hand isn’t the brand your doctor wrote on the slip. It’s probably a generic version - cheaper, just as effective, and often dispensed without you ever being asked. That’s because in 43 out of 50 U.S. states, pharmacists operate under presumed consent laws. These rules let them swap your brand-name drug for a generic without getting your explicit okay at the counter. It’s legal, it’s common, and most people never notice. But here’s the thing: not all substitutions are the same, and not all patients are okay with it.
How Presumed Consent Works in Real Life
Presumed consent doesn’t mean pharmacists can substitute any drug they want. It only applies to medications the FDA has rated as therapeutically equivalent - meaning they work the same way in your body as the brand name. These are marked with an "A" rating in the FDA’s Orange Book. For example, if your doctor prescribes Lipitor (atorvastatin), the pharmacist can give you the generic atorvastatin without asking. The active ingredient is identical. The fillers might be different, but the drug does the same job.
In states with presumed consent, the pharmacist assumes you’re okay with the switch unless you say otherwise. You don’t have to sign anything. You don’t have to answer a question. The system is built for speed. A 2022 study by the American Society of Health-System Pharmacists found that presumed consent cuts prescription processing time by about 1.7 minutes per script. That adds up to $2.8 billion saved in pharmacy labor costs each year across the U.S.
But here’s where it gets tricky: even though the system saves time and money, it doesn’t mean you’re always informed. In 31 states and Washington, D.C., pharmacists are required to notify you after the substitution - usually by adding a sticker to the bottle or handing you a paper notice. In the other 12 presumed consent states, there’s no legal obligation to tell you at all. You might never know you got a generic unless you check the label or notice the price dropped.
Where the Rules Change - And Why It Matters
Not every state plays by the same rules. Nineteen states have mandatory substitution laws - meaning pharmacists must switch to the generic unless the doctor writes "dispense as written" or "no substitution." In those places, the system is even more automated. But in 31 states, substitution is optional. The pharmacist can choose to give you the brand name if they think it’s better for you - or if you ask for it.
The biggest differences come with certain types of drugs. For most medications - blood pressure pills, antibiotics, cholesterol drugs - generics work just fine. But for drugs with a narrow therapeutic index (NTI), even tiny differences in how the body absorbs the drug can cause problems. These include seizure medications like phenytoin, thyroid drugs like levothyroxine, and blood thinners like warfarin.
Fifteen states, including Tennessee, Hawaii, and New York, have special rules for NTI drugs. In those places, pharmacists can’t substitute without your explicit permission. Some states require the doctor to write "brand necessary" on the prescription. Others require the pharmacist to call you first. The Epilepsy Foundation documented 178 cases of breakthrough seizures between 2018 and 2022 linked to generic switches in antiepileptic drugs. That’s not common - but it’s enough to make regulators pause.
And then there’s the newer class of drugs: biosimilars. These are generic versions of complex biologic drugs like Humira or Enbrel. Unlike small-molecule generics, biosimilars aren’t exact copies. They’re highly similar, but manufacturing them is like recreating a Swiss watch from a blueprint - close, but not identical. Only six states allow automatic substitution of interchangeable biosimilars without patient consent. Four states - North Carolina, Oklahoma, Pennsylvania, and Texas - ban it entirely. The rest are still figuring it out.

What Patients Really Think
Most people don’t mind the switch. On Drugs.com, 68% of the 1,243 comments about generic substitution were positive. Common feedback: "Saved me $45 a month," or "I didn’t even notice a difference." For Medicare beneficiaries, the savings are huge - an average of $627 per person each year.
But the negative experiences stick out. James T. from Tennessee posted in March 2023: "My seizure medication stopped working after substitution." His story isn’t rare. In states without NTI protections, patients with epilepsy, thyroid disorders, or heart conditions are at higher risk of adverse events after a switch.
Pharmacists report mixed results too. One Reddit user from Ohio, u/MedReviewSpecialist, wrote: "95% of patients don’t notice. The 5% who do? They get angry. They think we’re cutting corners." That’s the trust problem. Even when the science says generics are safe, patients feel like they’ve been left out of the decision. That’s why notification matters - not just as a legal requirement, but as a way to build confidence.
What You Can Do - Even in Presumed Consent States
You don’t have to accept a substitution just because the law allows it. Here’s how to take control:
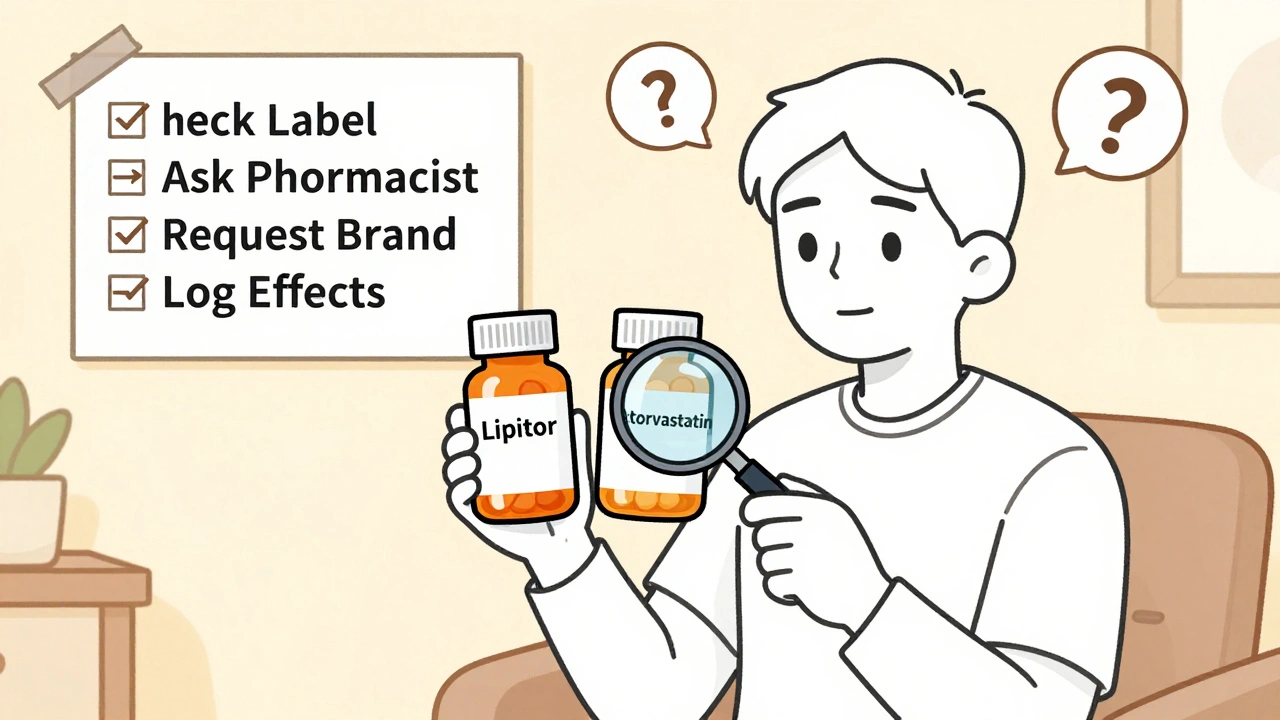
- Check your prescription label. Look for the drug name. If it says "atorvastatin" instead of "Lipitor," you’ve been switched.
- Ask your pharmacist. Say: "Was this switched from the brand?" They’re required to answer. In states with notification rules, they should already have told you.
- Request the brand. You have the right to ask for the original drug. If your insurance covers it, they’ll fill it. If not, you’ll pay more - but you’ll know you’re getting exactly what your doctor prescribed.
- Ask your doctor to write "dispense as written" or "no substitution." This legally blocks the pharmacist from switching. It’s especially important if you’re on an NTI drug or have had bad reactions to generics before.
- Keep a log. Write down every time you get a generic. Note any side effects or changes in how you feel. This helps you spot patterns and talk to your doctor.
The Bigger Picture - Why This System Exists
Presumed consent laws weren’t created to trick patients. They were created to save money - and they’ve done that well. Generic drugs make up 90% of all prescriptions dispensed in the U.S. but only cost 15% of what brand-name drugs do. Over the past decade, they’ve saved the healthcare system $1.68 trillion, according to the Association for Accessible Medicines.
That money goes to patients, insurers, and taxpayers. It means fewer people skip doses because they can’t afford their meds. It means more people can get treated for chronic conditions. But efficiency shouldn’t come at the cost of transparency.
The real challenge isn’t whether generics work - they do. The challenge is making sure patients understand what’s happening and feel included in the decision. Right now, the system is optimized for speed and cost, not communication.
What’s Next?
More states are starting to tighten rules around NTI drugs and biosimilars. New York passed a law in March 2023 requiring electronic records of every substitution. California expanded its notification rules for biosimilars in 2022. The Uniform Law Commission is working on a "Model State Substitution Act" that could bring more consistency across states.
But until federal standards are set, you’re stuck navigating 51 different sets of rules. The best defense? Stay informed. Ask questions. Know your rights. And don’t assume that just because a substitution is legal, it’s always the right choice for you.
Can my pharmacist switch my medication without telling me?
In 43 states and Washington, D.C., yes - under presumed consent laws, pharmacists can substitute generic drugs without asking. But 31 of those states require them to notify you afterward, usually with a sticker or paper notice. In the other 12 presumed consent states, there’s no legal requirement to inform you at all. Always check your prescription label to be sure.
Are generic drugs really the same as brand-name drugs?
For most medications, yes. The FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration as the brand. They must also prove they work the same way in the body. However, inactive ingredients (fillers, dyes, preservatives) can differ, which sometimes causes issues for people with allergies or sensitivities. For drugs with a narrow therapeutic index - like seizure or thyroid meds - even small differences in absorption can matter.
What if I don’t want a generic?
You have the right to refuse a substitution. Ask your pharmacist for the brand-name drug. If your insurance covers it, they’ll fill it. If not, you’ll pay more out of pocket. You can also ask your doctor to write "dispense as written" or "no substitution" on your prescription. This legally prevents the pharmacist from switching it.
Which drugs should never be substituted?
Drugs with a narrow therapeutic index (NTI) are the main concern. These include antiepileptics (phenytoin, carbamazepine), thyroid hormones (levothyroxine), blood thinners (warfarin), and some psychiatric drugs. In 15 states, pharmacists must get your explicit consent before substituting these. If you’re on one of these drugs, always confirm with your pharmacist and consider asking your doctor to block substitution.
Do biosimilars follow the same rules as generics?
No. Biosimilars are generic versions of complex biologic drugs like Humira or Enbrel. They’re not exact copies, so substitution rules are stricter. Only six states allow automatic substitution without patient consent. Four states ban it entirely. In most states, pharmacists must get your permission before switching you to a biosimilar. Always ask if your new medication is a biosimilar - and whether you’ve been switched.







10 Comments