When your prescription runs out and the pharmacy says they can’t restock, it’s not a glitch-it’s a systemic failure. Drug shortages aren’t rare accidents anymore. They’re predictable outcomes of supply chains built for efficiency, not safety. In 2025, over 200 essential medications in the U.S. faced recurring shortages, from antibiotics to insulin and life-saving injectables. The root cause? A global system that outsourced too much, trusted too few, and planned for too little. But here’s the truth: drug shortages can be prevented. It doesn’t take a miracle. It takes smarter infrastructure, better planning, and real investment. And it’s already happening in places that refuse to wait for the next crisis.
Why Your Medicine Isn’t Available
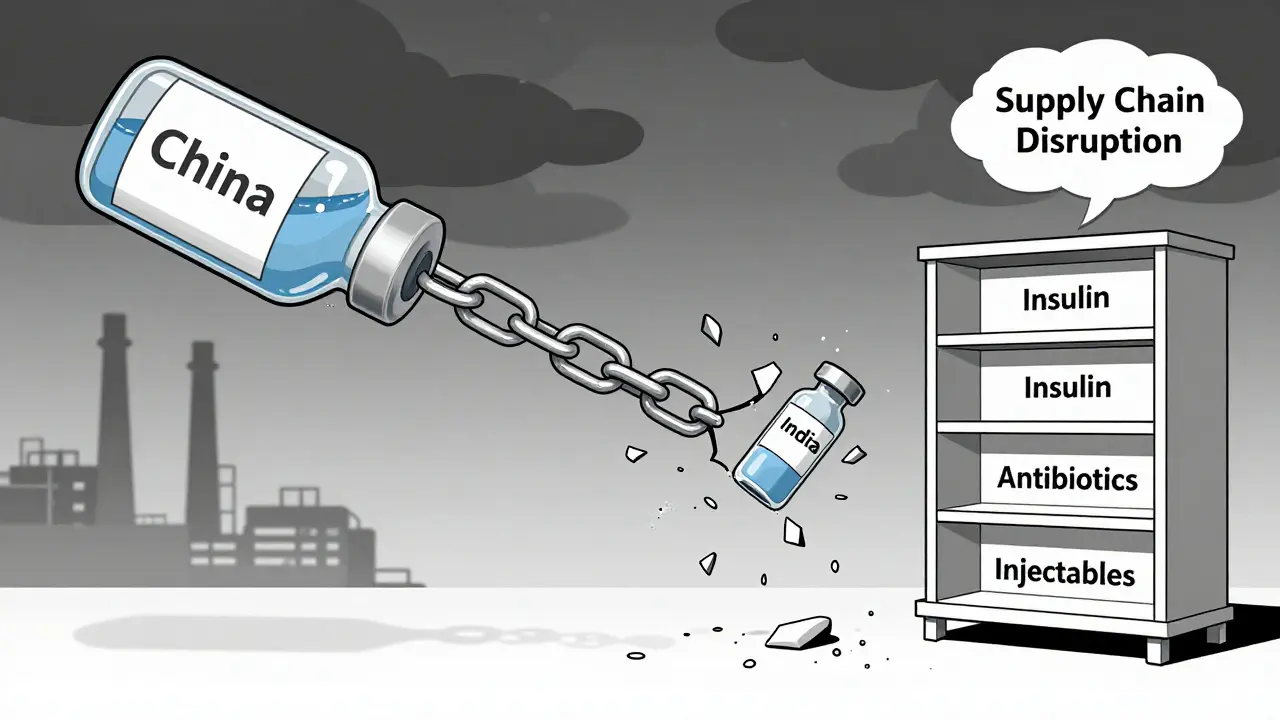
Most people think drug shortages happen because factories break down or raw materials run out. That’s only part of it. The real problem is concentration. About 80% of the active ingredients in U.S. drugs come from just two countries: China and India. India alone makes 23% of all APIs (active pharmaceutical ingredients), while China handles 45%. That’s not diversity-it’s a single point of failure with two names. When floods hit a manufacturing zone in India, or trade tensions freeze exports from China, the ripple effect hits hospitals, pharmacies, and patients within weeks.
It gets worse. Only 28% of essential medicine APIs are made in the U.S. today. Sterile injectables? Just 12% domestic. Antibiotics? 17%. That means when global supply chains stutter, the U.S. doesn’t have a backup. And because many drugmakers run lean inventories-sometimes just 10-15 days of stock-there’s no buffer. No safety net. One delay, one shipment held at customs, one power outage, and patients go without.
What Resilience Actually Looks Like
Resilience isn’t a buzzword. It’s a set of concrete actions. The Mathematica Inc. report from 2023 defines it clearly: the ability to anticipate, prepare for, respond to, and recover from disruptions while keeping critical drugs flowing. That means three things: preparedness, response, and recovery.
Preparedness starts with mapping your supply chain-not just Tier 1 suppliers, but Tier 12. Leading companies now track dependencies across 12 to 15 layers of suppliers. Why? Because a chemical solvent used in your pill might come from a plant in Poland, made from a compound sourced in Vietnam, shipped through a port in Singapore. One breakdown anywhere, and your entire production line stops. Mapping these links lets you spot hidden risks before they explode.
Response means having options. The most resilient companies dual-source at least 70-80% of their critical ingredients. That means if Supplier A in India can’t deliver, Supplier B in Ireland or Mexico steps in. No single supplier holds more than 30% of your volume for essential drugs. And they keep 60 to 90 days of inventory for high-risk medications-not weeks. That’s not hoarding. That’s insurance.
Recovery isn’t about fixing damage after it happens. It’s about rebuilding faster. That’s where new manufacturing tech comes in. Traditional batch production takes months to set up, requires huge facilities, and wastes 15-20% of material. Continuous manufacturing changes that. It’s like a流水线 for drugs-24/7, compact, efficient. One facility using this tech uses 30-40% less space, cuts energy use by 20-25%, and slashes waste by 15-20%. And it can be built in 12-18 months instead of 3-5 years.
The Tech That’s Changing the Game
AI and automation aren’t just for self-driving cars. In pharma, they’re keeping pills on shelves.
AI-powered systems now predict disruptions with 85-90% accuracy up to 90 days ahead. They analyze weather patterns, port congestion, political unrest, even supplier financial health. One U.S. manufacturer started using this tool in 2024. In early 2025, it got a heads-up that a key API supplier in Gujarat was about to shut down for maintenance. They shifted production to a backup facility in Canada two weeks before the shutdown. No shortage. No panic.
Blockchain traceability is another quiet revolution. Instead of paper logs and faxed certificates, every batch of API is tracked from raw material to final pill. Pilots in Europe and the U.S. showed a 70-75% drop in counterfeit drugs. Why? Because every step is verifiable. No one can slip in fake ingredients. No one can hide delays.
And then there’s modular manufacturing. Think shipping containers turned into drug factories. A single 20-foot container can produce 50kg to 2,000kg of API per year. It’s portable, scalable, and can be set up in rural areas or near hospitals. The U.S. government is testing these in five states. Imagine a hospital in rural Kentucky that can make its own insulin locally-no shipping, no delays, no foreign dependence.
Who’s Doing It Right
Not every company is waiting for a crisis. A mid-sized U.S. generics maker in Ohio started rebuilding its supply chain in 2022. They mapped every supplier. They dual-sourced 80% of their critical APIs. They invested in a continuous manufacturing unit. They kept 90 days of inventory for their top 10 drugs. By 2024, they had zero shortages. In 2025, they won a federal contract to supply 12 essential antibiotics to the national stockpile. Their cost of goods rose 8%, but their revenue grew 22% because hospitals trusted them.
Another example: a large pharmaceutical firm in Maryland partnered with a logistics tech startup to build a real-time supply chain dashboard. It shows inventory levels, shipping delays, and supplier risk scores on a single screen. When a hurricane hit Puerto Rico in early 2025, the system flagged that 30% of their sterile injectables came through that port. Within hours, they rerouted shipments through Florida and activated backup suppliers. No patient went without.
These aren’t outliers. They’re the companies that spent 8-10% of their supply chain budget on resilience. The industry average? 5%. The difference? Companies with high resilience spending saw 23% higher operational continuity during disruptions-and avoided $14.7 million in lost revenue per major event.
The Cost of Inaction
Some say, “Why spend more? The market will fix itself.” But the market doesn’t fix itself when lives are on the line. The U.S. government estimates that drug shortages cost the healthcare system over $20 billion annually in emergency care, delayed treatments, and hospital readmissions. Patients die. Nurses scramble. Doctors prescribe alternatives that don’t work as well.
And the cost of resilience? It’s real. Continuous manufacturing costs $50-$150 million per facility-3 to 5 times more than a traditional plant. Dual-sourcing adds 8-12% to the cost of goods. But here’s the math: for every $1 spent on resilience, companies get $1.80 back in avoided losses within 3 years. That’s not a cost. It’s a return.
What’s worse than spending? Being unprepared. In 2024, a shortage of a common antibiotic led to a 300% price spike. Hospitals paid $400 per vial instead of $40. Patients got sicker. Emergency rooms filled up. The company that made the drug? They didn’t lose money-they lost trust. And trust doesn’t come back with a press release.
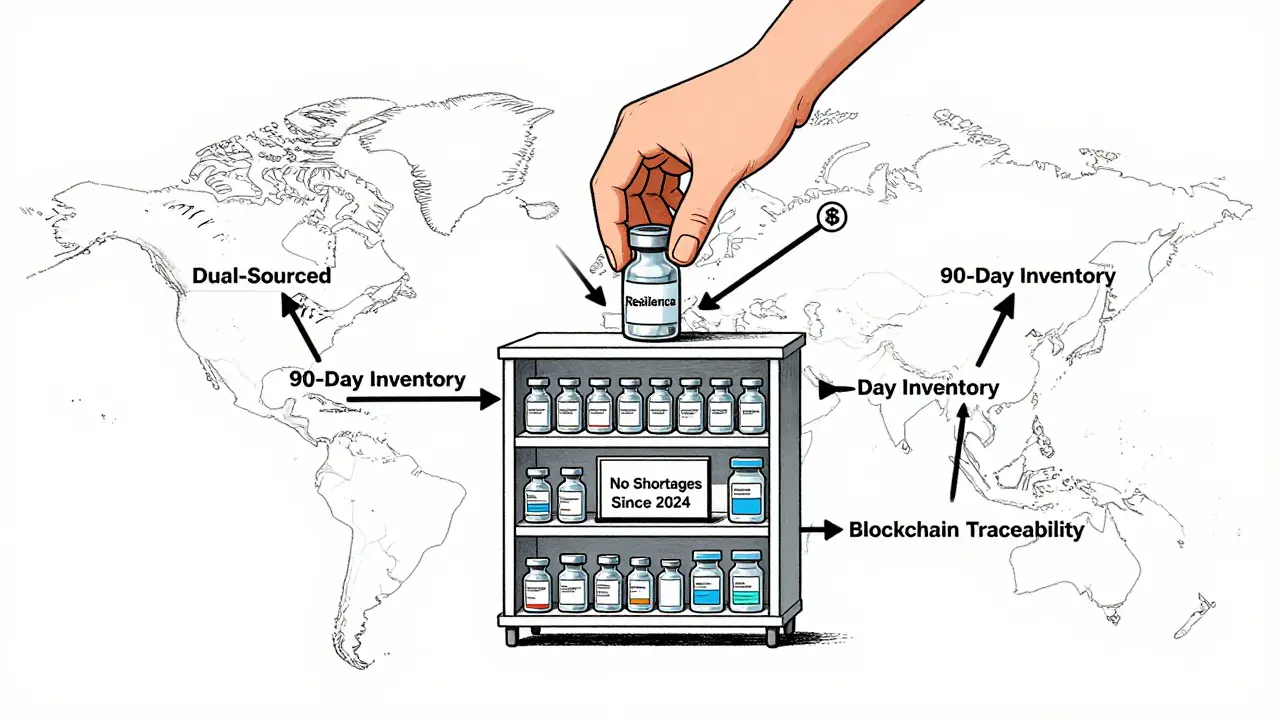
What Needs to Change
Government action matters. The 2025 Executive Order to fill the Strategic Active Pharmaceutical Ingredients Reserve is a step. It aims to stockpile 90-day supplies of 150 critical drugs by 2027. That’s good. But stockpiles are reactive. We need proactive change.
Regulators need to move faster. The FDA has approved only 12 continuous manufacturing facilities since 2000. In 2025, they cut approval time from 3 years to 18 months. That’s progress. But they need to make it faster, simpler, and cheaper for smaller companies to adopt.
Workforce shortages are another silent crisis. By 2027, the U.S. will need 250,000 more skilled manufacturing workers. No one’s training them. No one’s offering scholarships. No one’s building pipelines from community colleges into pharma plants.
And global trade can’t be ignored. Tariffs won’t bring all manufacturing home. You can’t make complex APIs in a vacuum. The answer isn’t “make everything here.” It’s “make smart things here, and build trusted networks everywhere else.”
The Path Forward
Building resilience isn’t optional anymore. It’s survival. Here’s what works:
- Map your supply chain to Tier 12. Know who makes what, where, and how.
- Dual-source 70-80% of critical ingredients. No single supplier should control your fate.
- Hold 60-90 days of inventory for essential medicines. Not 10 days. Not 30. 60-90.
- Invest in continuous manufacturing where you can. It’s faster, cheaper long-term, and more reliable.
- Use AI and blockchain to predict risks and track every batch.
- Build regional networks-North America, Europe, Southeast Asia. Don’t rely on one continent.
- Get executive buy-in. Resilience fails without leadership. Companies with strong sponsorship see 3.2x higher success rates.
It’s not about being perfect. It’s about being prepared. The next drug shortage isn’t a question of if-it’s when. The question is: will your pharmacy be ready?






14 Comments