Personalized Levonorgestrel Dosing Calculator
Personalized Levonorgestrel Dosing Tool
Based on the latest research in personalized contraception, this tool estimates a more effective levonorgestrel dose tailored to your individual needs. Your input helps demonstrate how innovative delivery systems and pharmacogenomics can improve contraceptive effectiveness while minimizing side effects.
When you hear Levonorgestrel BP is the formulation of the synthetic progestin levonorgestrel that meets the standards set by the British Pharmacopoeia (BP), you probably think of the Monday‑after pill or low‑dose birth control pills. Those products have saved millions of lives, but scientists are already sketching the next generation of this hormone. From nanocarriers that release the drug over days to 3‑D printed tablets that can be customized for each user, the future promises faster, safer, and more personalized options.
Where Levonorgestrel Stands Today
Levonorgestrel works by stopping ovulation and thickening cervical mucus, making it hard for sperm to reach an egg. In the United Kingdom and many other markets, the drug is produced under the strict guidelines of the British Pharmacopoeia, ensuring consistency in potency and purity. Current products fall into two main categories: oral emergency contraceptives (often called the "morning‑after pill") and low‑dose combined oral contraceptives (COCs) that also contain estrogen.
Despite their success, existing formulations have drawbacks. Oral tablets can cause nausea, timing is critical, and the fixed dose doesn’t account for individual metabolism differences. That's why researchers are looking for ways to improve delivery, reduce side effects, and broaden access.
Regulatory Landscape Shaping Innovation
The U.S. Food and Drug Administration (FDA) and the World Health Organization (WHO) set the safety bar for new contraceptive technologies. Both agencies have recently issued guidance encouraging the development of patient‑centric drug delivery systems, especially those that can lower systemic exposure while maintaining efficacy. Their openness to novel dosage forms-like dissolvable films or implants-means innovators have a clearer path to market, provided they meet rigorous pharmacokinetic and toxicology standards.
Innovation #1: Nanoparticle Drug Delivery
Nanoparticles can hide the drug inside tiny carriers that release levonorgestrel slowly over a week or more. In pre‑clinical trials, Nanoparticle drug delivery systems have shown a 30% reduction in nausea compared to standard tablets while keeping hormone levels within the therapeutic window.
These carriers are usually made of biodegradable polymers like PLGA (poly‑lactic‑co‑glycolic acid). Once injected subcutaneously, the particles slowly degrade, letting the drug drip out. The result: a single injection could replace a whole week’s worth of pills, making adherence almost guaranteed.
Innovation #2: 3‑D Printed Personalized Tablets
3‑D printing, also known as additive manufacturing, lets pharmacists create tablets with precise layer‑by‑layer dosing. By adjusting the geometry of each tablet, manufacturers can tailor the release profile to an individual’s weight, age, or metabolic rate. Early pilots at a university hospital used 3D printed tablets to cut the dose for a 55‑kg patient by 20% without sacrificing contraceptive efficacy.
Beyond dose customization, this technology reduces waste. A single printer can produce small batches on demand, cutting the need for large inventories and lowering the carbon footprint of drug manufacturing.
Innovation #3: Combination with Long‑Acting Reversible Contraception (LARC)
Researchers are exploring hybrid systems that pair levonorgestrel with a Intrauterine device (IUD) or a subdermal implant. The idea is to provide immediate hormonal coverage while the device slowly releases the hormone over years. A recent trial combined a low‑dose levonorgestrel oral pill with a copper‑IUD, reporting a 99.8% pregnancy‑preventing rate and a shorter period of post‑insertion spotting.
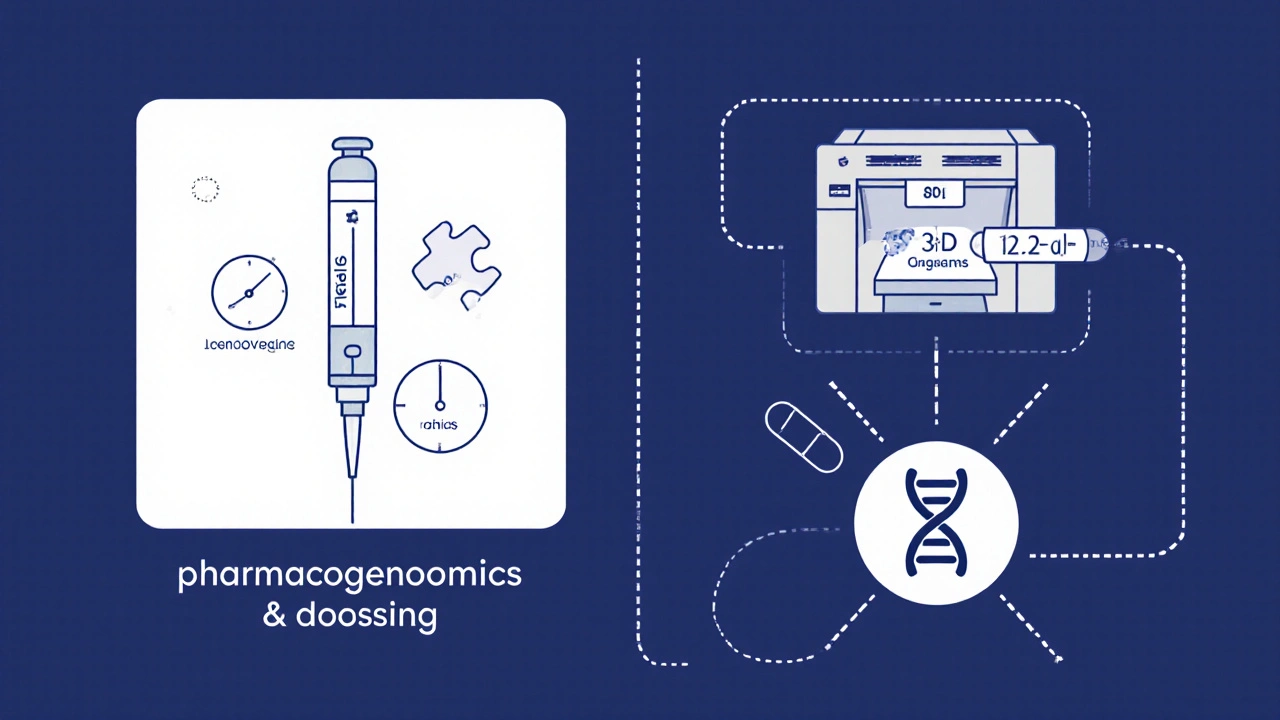
Innovation #4: Pharmacogenomics‑Driven Dosing
People metabolize levonorgestrel at different speeds, largely because of genetic variations in the CYP3A4 enzyme. Pharmacogenomics tests can identify fast or slow metabolizers before prescribing. In a small cohort, genotype‑guided dosing reduced breakthrough bleeding episodes by 40% compared with standard dosing.
Integrating a simple cheek‑swab test into pharmacy workflows could soon make personalized levonorgestrel dosing a routine part of family‑planning visits.
Innovation #5: Sustainable Manufacturing Practices
The pharmaceutical industry is under pressure to lower its environmental impact. Green chemistry approaches, like solvent‑free synthesis and continuous flow reactors, are being applied to levonorgestrel production. A pilot plant in Europe reported a 25% reduction in waste water and a 15% cut in energy use while maintaining BP‑grade purity.
These greener methods not only benefit the planet but also can lower costs, making the drug more affordable in low‑resource settings.
Potential Clinical Impact
- Improved adherence: Long‑acting and slow‑release formats cut the need for daily pills.
- Reduced side effects: Nanocarriers and personalized dosing lower systemic exposure.
- Broader access: On‑demand 3‑D printing and sustainable manufacturing can lower prices.
- Enhanced safety: Pharmacogenomic screening prevents over‑ or under‑dosing.
Together, these improvements could shift levonorgestrel from a rescue option to a mainstream, patient‑centric contraceptive choice.
Challenges on the Road to Adoption
Regulatory approval remains the biggest hurdle. Nanoparticle formulations must demonstrate long‑term safety, especially regarding polymer degradation products. Likewise, widespread pharmacogenomic testing requires clear reimbursement policies and data‑privacy safeguards.
Cost is another factor. While 3‑D printing reduces inventory waste, the upfront equipment investment can be steep for small pharmacies. Partnerships between manufacturers and health systems could spread the expense.

Roadmap: What to Expect in the Next Five Years
- 2025‑2026: Phase‑I/II trials for levonorgestrel‑loaded nanoparticles in Europe and North America.
- 2026‑2027: FDA and EMA issue guidance on nanocarrier‑based hormonal contraceptives.
- 2027‑2028: Commercial launch of the first 3‑D printed personalized levonorgestrel tablet in select clinics.
- 2028‑2029: Integration of pharmacogenomic testing into routine pharmacy practice in the UK and Canada.
- 2029‑2030: Sustainable manufacturing becomes the default for major levonorgestrel producers, driving down price points globally.
If these milestones hold, patients could choose from at least three novel levonorgestrel options by the start of the next decade.
Quick Takeaways
- Nanoparticle carriers promise fewer side effects and weekly dosing.
- 3‑D printing enables dose customization and reduces waste.
- Pharmacogenomics can fine‑tune therapy for individual metabolism.
- Regulators are increasingly supportive of innovative delivery systems.
Frequently Asked Questions
How does nanotechnology improve levonorgestrel's safety?
Nanoparticles encapsulate the hormone, allowing a slower, more controlled release. This means lower peak blood concentrations, which reduces nausea and other estrogen‑related side effects while keeping contraceptive efficacy high.
Can I get a 3‑D printed levonorgestrel tablet at my local pharmacy?
Most pharmacies don’t have the equipment yet. Pilot programs in specialist clinics are expected to expand by 2028, after regulatory clearance and cost‑reduction studies prove viability.
Is pharmacogenomic testing required before using levonorgestrel?
It isn’t required today, but it can help tailor the dose for people who metabolize the drug unusually fast or slow. As guidelines evolve, many clinicians may recommend a simple genetic screen as part of family‑planning visits.
Will these innovations increase the cost of contraceptives?
Initial rollout may be pricier due to new technology, but scale‑up, sustainable manufacturing, and reduced waste are expected to bring prices down over the next five years.
How do regulatory agencies evaluate new levonorgestrel delivery systems?
They require a full pharmacokinetic profile, demonstration of non‑inferior contraceptive efficacy, and safety data on the carrier material (e.g., polymer degradation products). Guidance documents released in 2025‑2026 are already streamlining the pathway for nanocarrier and 3‑D printed formulations.
Conclusion: A New Era for a Classic Hormone
The next wave of levonorgestrel BP innovations aims to make contraception more convenient, personalized, and environmentally friendly. By embracing nanotech, 3‑D printing, and genetic insights, the hormone that’s saved millions could become even more effective and user‑friendly. Keep an eye on regulatory updates and emerging clinical trial results - the future of this familiar drug is shaping up to be anything but ordinary.







9 Comments