Medication Costs: How Coupons, Generics, and Prior Authorizations Affect Your Pocket in 2026
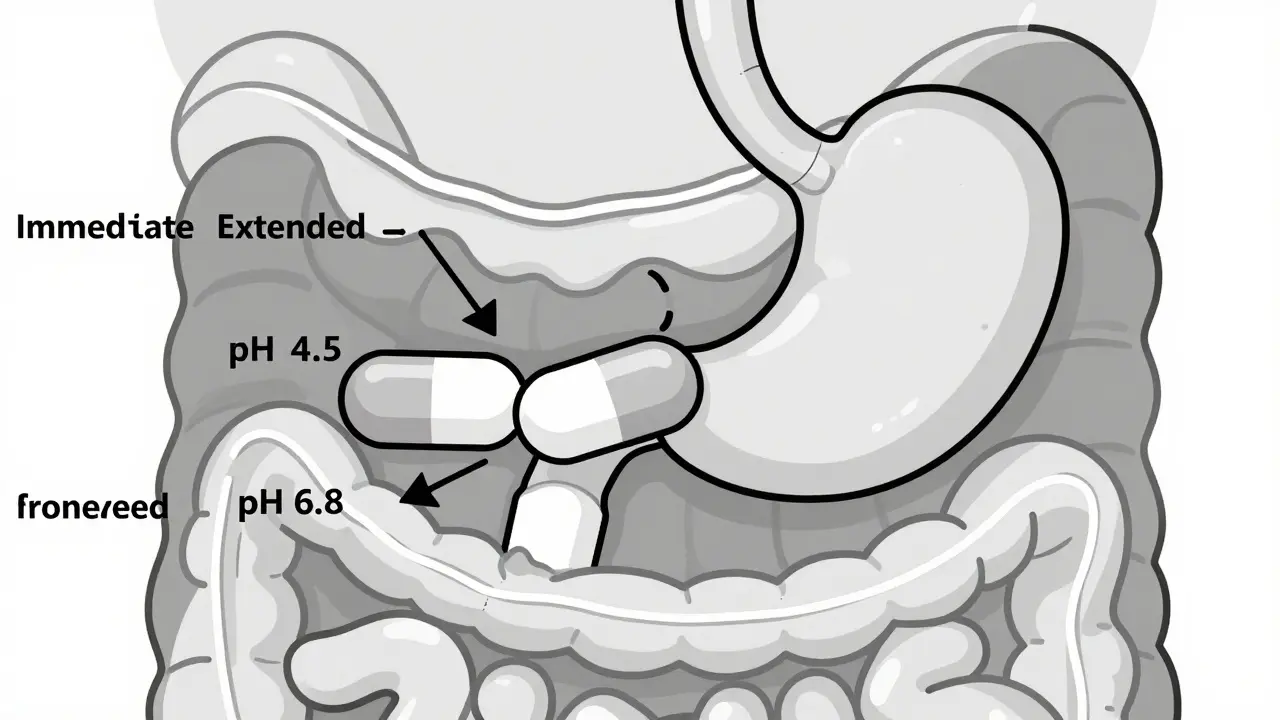
Feb, 1 2026In 2026, medication costs are changing fast. Learn how generics, coupons, and prior authorizations affect your out-of-pocket bills-and what you can do to save money on prescriptions under the new Medicare rules.