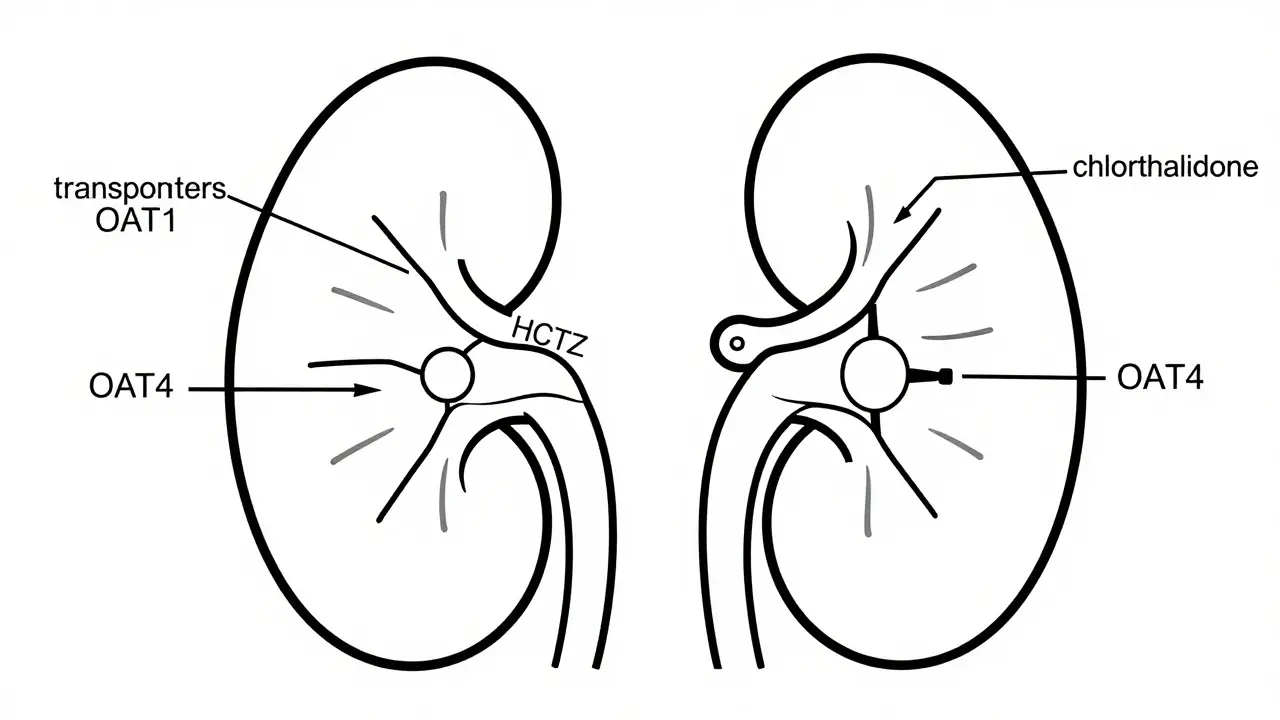
Thiazide diuretics like hydrochlorothiazide are common blood pressure meds but can raise uric acid and trigger gout. Learn who's at risk, how it happens, and what alternatives exist.
Category: Medications - Page 2
Medication Costs: How Coupons, Generics, and Prior Authorizations Affect Your Pocket in 2026
Feb, 1 2026In 2026, medication costs are changing fast. Learn how generics, coupons, and prior authorizations affect your out-of-pocket bills-and what you can do to save money on prescriptions under the new Medicare rules.
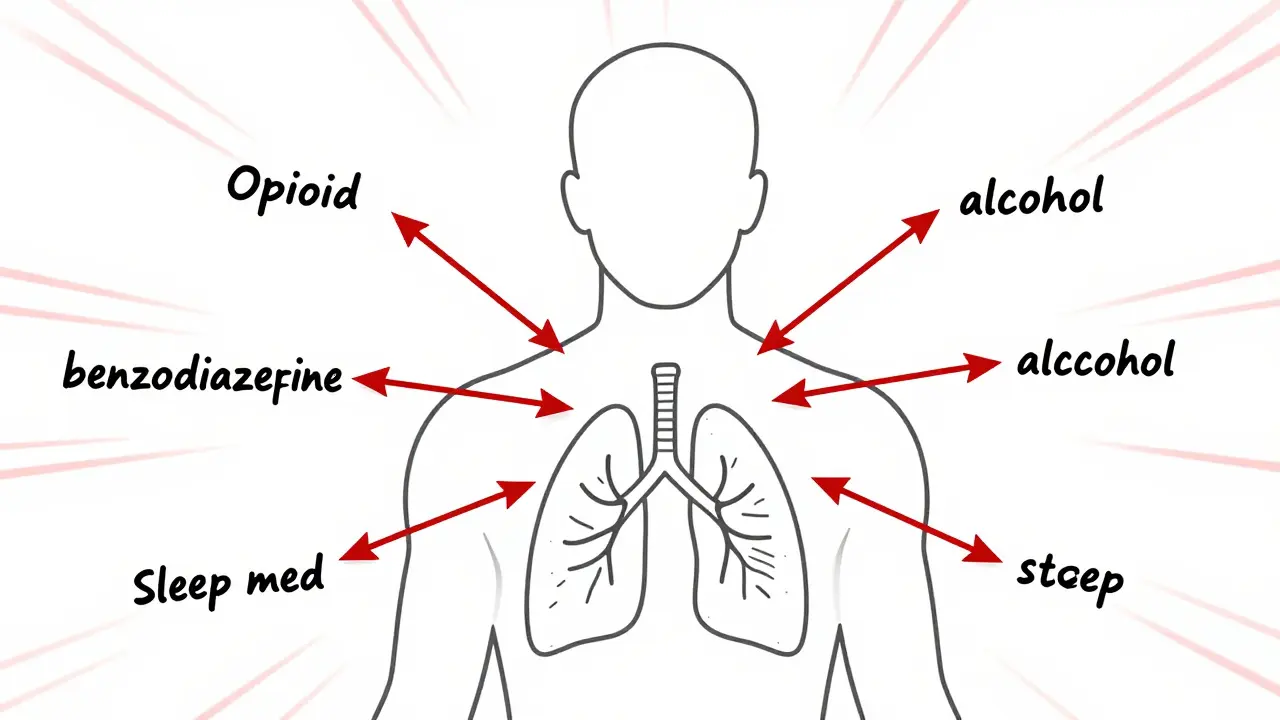
Combining sedatives like opioids, benzodiazepines, and alcohol can cause deadly respiratory depression. Learn the risks, common dangerous mixes, and what to do if you're taking multiple CNS depressants.
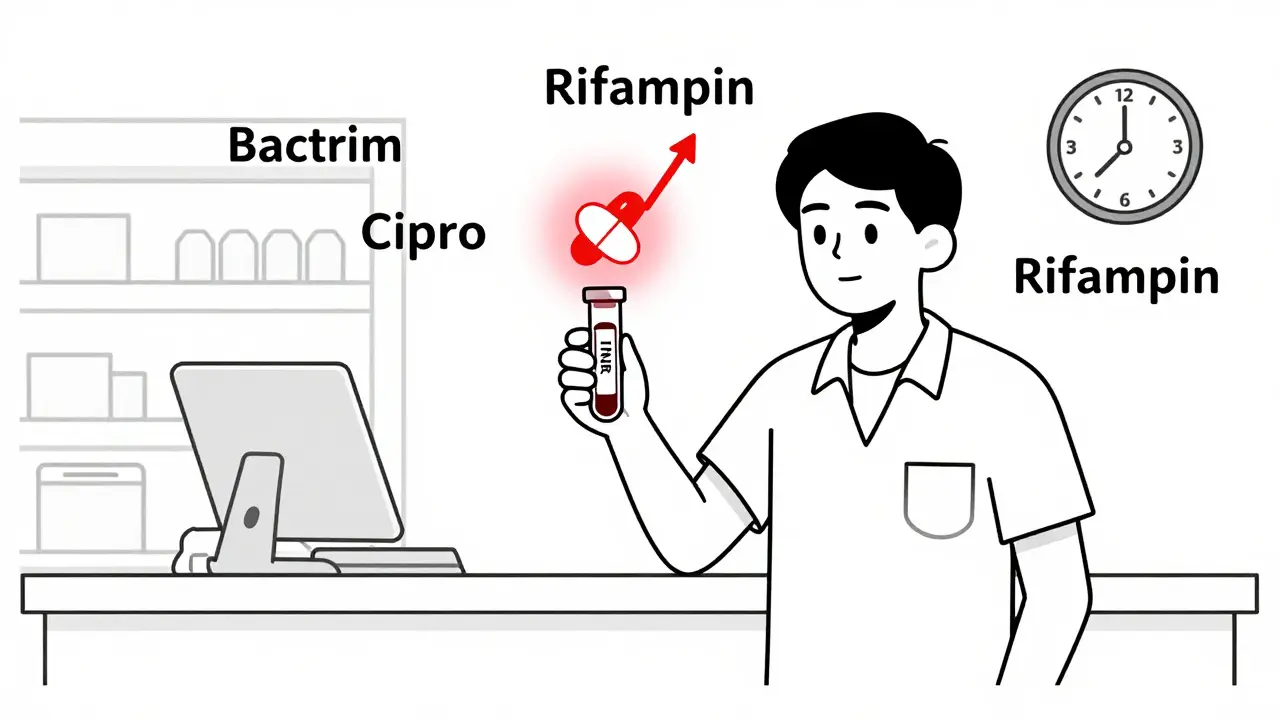
Warfarin and antibiotics can dangerously interact, raising your risk of bleeding. Learn which antibiotics are high-risk, when to check your INR, and how to stay safe without stopping your blood thinner.
Behind-the-counter medications require pharmacist consultation but no prescription. Learn what drugs are restricted in Australia, why they’re controlled, and how to navigate the process without hassle.
The ANDA process is the legal pathway for generic drug approval in the U.S., governed by the Hatch-Waxman Act. It requires bioequivalence, identical formulation, and strict manufacturing standards to ensure safe, affordable alternatives to brand-name drugs.
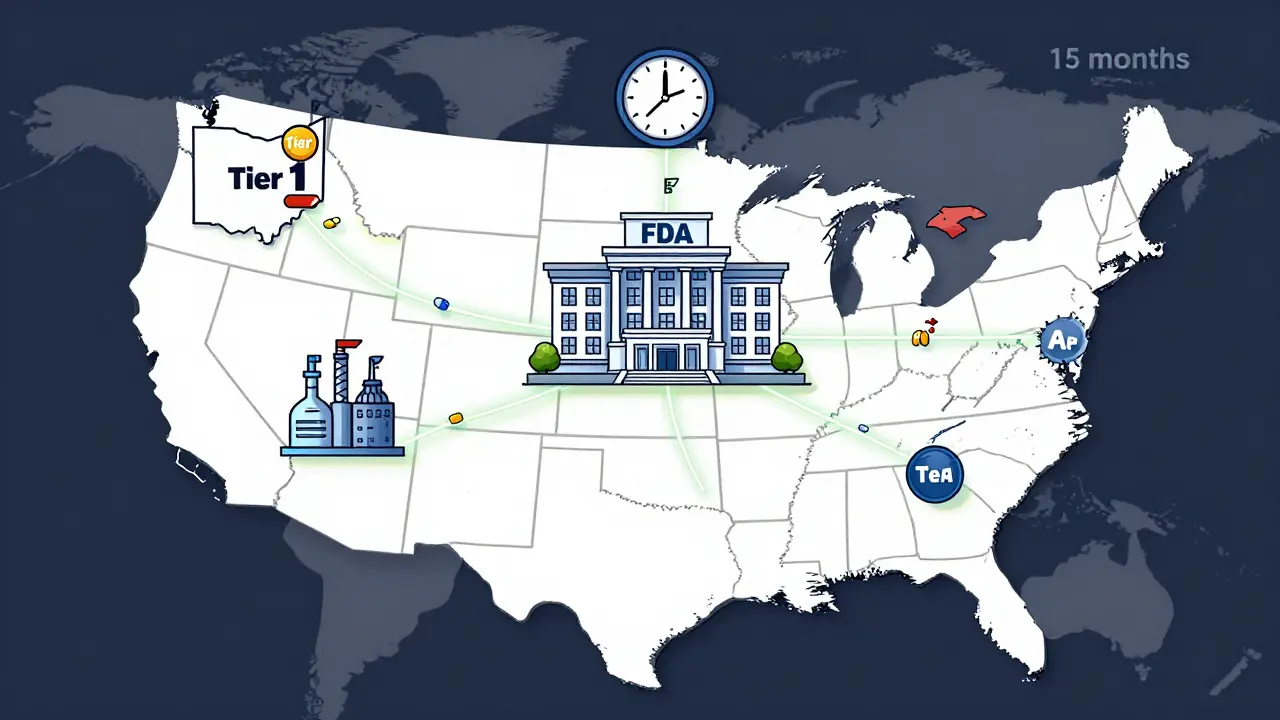
The FDA's 2023-2025 updates to generic drug approvals prioritize U.S.-made medications to fix supply chain gaps. Learn how the new pilot program affects drug availability, pricing, and manufacturing.
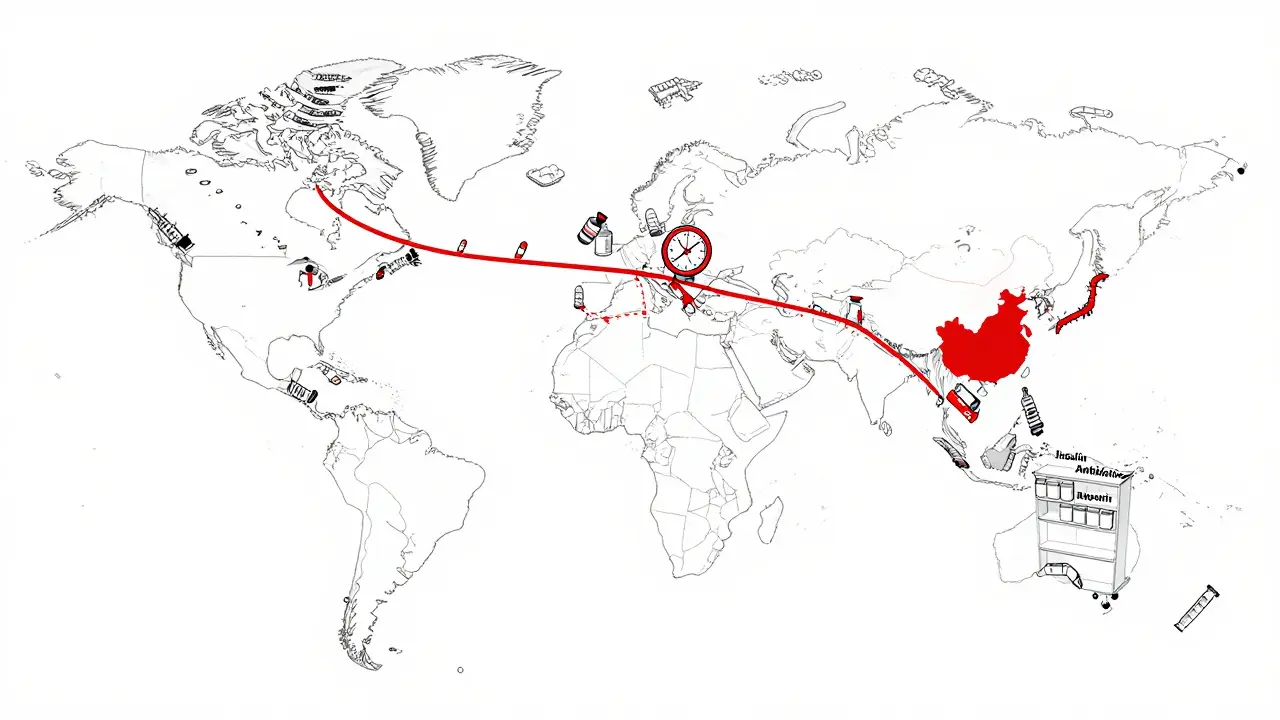
International Supply Chains and Drug Shortages: Why Foreign Manufacturing Is Putting Medicines at Risk
Jan, 20 2026Dependence on foreign manufacturing for pharmaceuticals is causing widespread drug shortages. Learn how China and India supply most of the world's active ingredients-and why diversifying supply chains is critical for patient safety.
Evergreening lets pharmaceutical companies extend drug monopolies with minor changes, blocking cheaper generics and keeping prices high. Learn how it works and why it hurts patients.
Generic drugs save the U.S. over $330 billion a year, but brand manufacturers face massive revenue losses when patents expire. Learn how patent cliffs, pay-for-delay deals, and PBM pricing distortions shape the real cost of medicine.