Decongestants and Heart Disease: What You Need to Know About Blood Pressure and Heart Risks
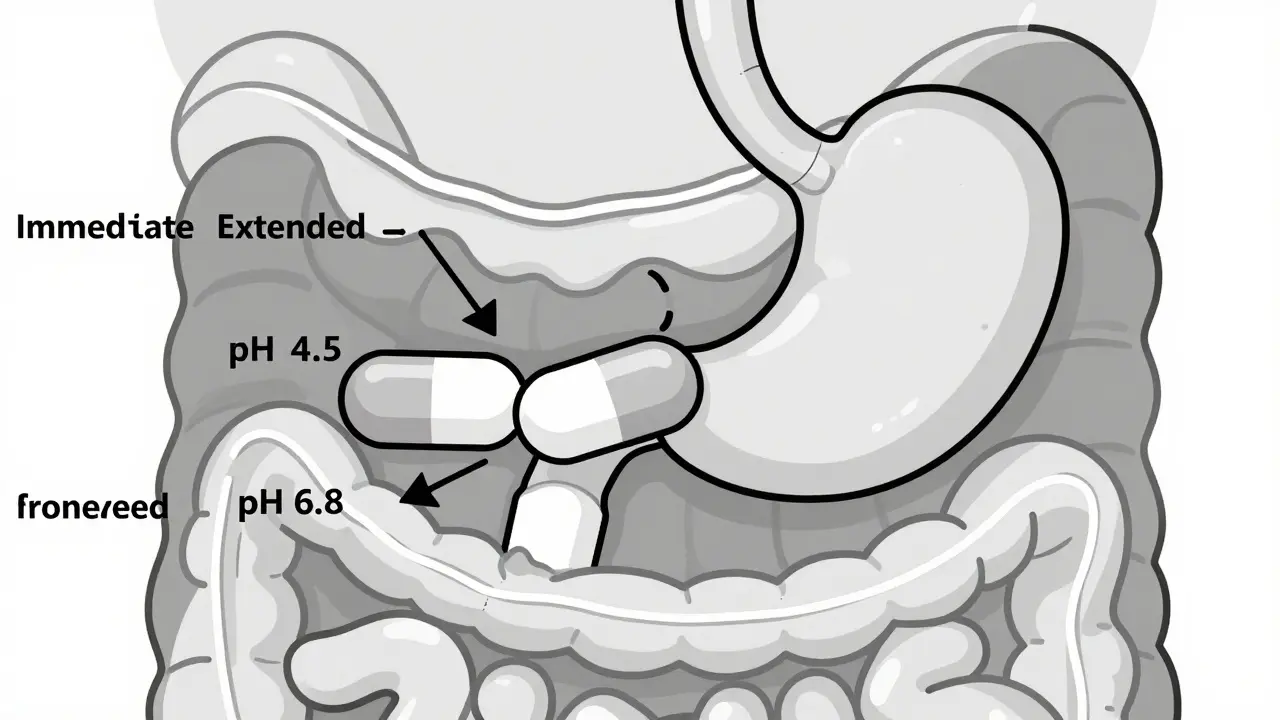
Dec, 31 2025Decongestants can raise blood pressure and trigger heart attacks or strokes in people with heart disease. Learn which ingredients to avoid, safer alternatives, and why even nasal sprays aren't always safe.